Ir/BiphPHOX-catalyzed asymmetric hydrogenation of 3-substituted 2,5-dihydropyrroles and 2,5-dihydrothiophene 1,1-dioxides†
Abstract
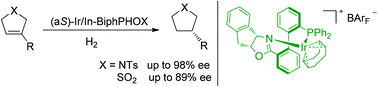
An efficient asymmetric hydrogenation of 3-substituted 2,5-dihydropyrroles using an Ir catalyst with an axially flexible chiral phosphine-oxazoline ligand was developed and chiral 3-substituted pyrrolidines could be prepared with excellent ee. 3-Substituted 2,5-dihydrothiophene 1,1-dioxides could also be hydrogenated by our catalyst, giving the desired products with moderate to high enantioselectivities.


 Please wait while we load your content...
Please wait while we load your content...