One-pot synthesis of N-heterocycles and enimino carbocycles by tandem dehydrative coupling–reductive cyclization of halo-sec-amides and dehydrative cyclization of olefinic sec-amides†‡
Abstract
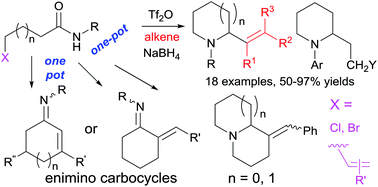
We report two efficient and versatile alkenylative cyclization methods for the one-pot synthesis of substituted pyrrolidine, piperidine, indolizidine, and quinolizidine ring systems, and enimino carbocycles, respectively. The first method consists of amide activation (Tf2O) induced dehydrative coupling of halogenated secondary amides with alkenes and the NaBH4 reduction triggered tandem cyclization reaction, while the second one features the Tf2O-promoted novel modes of extended Bischler–Napieralski cyclization reactions of olefinic secondary amides. Taking advantage of triflic anhydride (Tf2O) as the amide activating reagent, the hitherto failed two-step process for the construction of the quinolizidine ring system via intramolecular vinylogous Bischler–Napieralski cyclization has been realized in one pot. The first method was applied to the protecting-group-free one-pot synthesis of the cytotoxic natural product caulophyllumine B (5) and its bioactive derivatives, and to the synthesis of δ-coniceine and 6-styrylpiperidin-2-one. When ethyl vinyl ether and enamides were used as functionalized alkenes, saturated 1,3-amino-ether/amido products 4A1–4A3 were obtained in 73%–74% yields.


 Please wait while we load your content...
Please wait while we load your content...