Cobalt-catalyzed C–H activation and regioselective intermolecular annulation with allenes†
Abstract
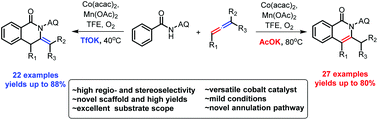
The cyclization reaction of allene has attracted considerable attention in recent years because of the unique reactivity and interesting modes of action in the process. However, rare examples have been reported on the annulation with allene via a C–H activation strategy. Here, we report a novel and efficient intermolecular annulation of N-(quinolin-8-yl)benzamide with allenes via cobalt-catalyzed C–H activation. This method provides a shortcut for the preparation of novel isoquinolin-1(2H)-one scaffolds, and both endo-cyclic and exo-cyclic isoquinolinones were respectively prepared in the presence of different bases. In addition, this C–H functionalization proceeds under mild conditions and tolerates a wide range of benzamide substrates, and reactions with terminal as well as internal allenes afforded the corresponding products in up to 88% yield. Furthermore, a new annulation pathway with allene is also presented.

 Please wait while we load your content...
Please wait while we load your content...