Formal enantioselective syntheses of oseltamivir and tamiphosphor†
Abstract
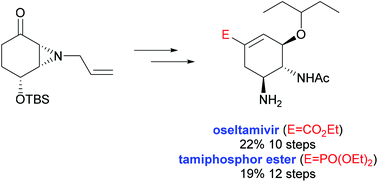
A new method for the synthesis of the influenza antiviral drugs oseltamivir and tamiphosphor starting from 4-hydroxycyclohexenone has been developed. Stereoselective aziridination of a protected 4-hydroxycyclohexenone was used as a key step and the aziridine formed was resolved enzymatically with a very high yield and enantiomeric excess. The subsequent conversion to oseltamivir and tamiphosphor diethyl ester in 10 or 12 additional steps and 22% or 19% yield from intermediate 7, respectively, is described.

 Please wait while we load your content...
Please wait while we load your content...