Gelatin based dynamic hydrogels via thiol–norbornene reactions†
Abstract
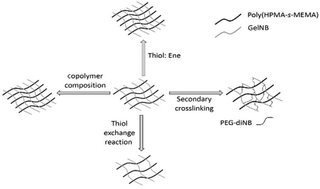
Gelatin based dynamic hydrogels have been synthesized by crosslinking norbornene functionalized gelatin with poly(2-hydroxypropyl methacrylate-s-mercaptoethyl methacrylate) (poly(HPMA-s-MEMA)) using radical mediated thiol–norbornene reactions. The poly(HPMA-s-MEMA) was prepared from a pyridyl disulfide functionalized poly(2-hydroxypropyl methacrylate-s-pyridyldisulfide ethylmethacrylate) (poly(HPMA-s-PDSEMA)) copolymer, which was synthesized using reversible addition fragmentation chain transfer (RAFT) polymerization. Subsequent reduction of the polymer using tris(2-carboxyethyl)phosphine (TCEP) afforded a copolymer with pendant thiol groups. The material properties of the hydrogels, including the swelling ratio and storage moduli (G′), were controlled by varying the thiol/ene molar ratio in the initial reaction mixture. Increases in material properties were observed with increasing thiol/ene molar ratio due to the formation of disulfide crosslinks in addition to the alkyl sulfide crosslinks. The dynamic behavior of these disulfides was considered to perform thiol exchange reactions with 2-mercaptoethanol to soften the hydrogel. Conversely, hydrogel stiffening was achieved through a secondary thiol–norbornene cross-linking between PEG-diNB and free thiols in the hydrogel. The gelatin based dynamic hydrogels demonstrated controllable stiffness over a 9.5 kPa–17.8 kPa range. These hydrogels are candidates for studying dynamic cell processes, for example fibrosis.


 Please wait while we load your content...
Please wait while we load your content...