Hollow particles are produced by the burying of sulfate end-groups inside particles prepared by emulsion polymerization of styrene with potassium persulfate as initiator in the absence/presence of a nonionic emulsifier†‡
Abstract
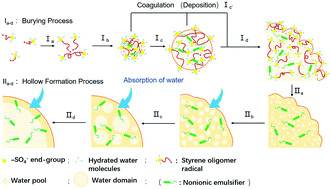
We prepared submicrometer-sized polystyrene (PS) particles by emulsion polymerization with/without polyoxyethylene nonylphenyl ether nonionic emulsifier (Emulgen 911), and examined how water absorption was affected by polymer end-groups derived from hydrophilic (ionic) or hydrophobic (nonionic) initiator and/or incorporated nonionic emulsifier when they were inside these particles. PS particles having sulfate end-groups, which were prepared using a potassium persulfate initiator with/without Emulgen 911, absorbed a certain amount of water inside them, whereas those having isobutyronitrile end-groups derived from 2,2′-azobis(isobutyronitrile) initiator did not. Absorption of water inside the particles caused by the incorporated Emulgen 911 alone was minimal in the absence of the sulfate end-groups. Considering that sulfate end-groups existing at the particle surface do not contribute to water absorption into the particles, these results indicate that sulfate end-groups did not only exist at the surface of the PS particles but were also buried inside the PS particles during the emulsion polymerization. This offers a clear explanation of a longtime enigma in (emulsifier-free) emulsion polymerization. Both the ionic end-groups buried in the particles and the nonionic emulsifier incorporated inside cooperate to absorb water, thus resulting in the formation of hollow PS particles.


 Please wait while we load your content...
Please wait while we load your content...