Toward alternating copolymerization of maleimide and vinyl acetate driven by hydrogen bonding†
Abstract
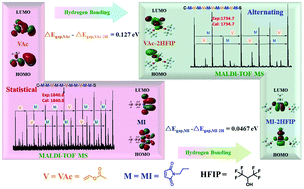
Herein, we report the solution copolymerization of N-propylmaleimide (MI) and vinyl acetate (VAc) in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) and 1,4-dioxane. The polymerization was carried out with a living radical method using 2-(ethoxycarbonothioyl) sulfanyl propanoate (EXEP) as the mediator. The copolymerization behaviour of this monomer pair was investigated in detail with different feeding ratios and at different temperatures. The reactivity ratio for such monomer pair in different conditions was determined. The structures of the copolymers were characterized by NMR and by MALDI-TOF mass spectra. The hydrogen-bonding interaction between MI and VAc with HFIP was simulated by the computational approach of quantum chemistry. This revealed that fluoroalcohols were quite effective in affording the copolymers with controlled molecular weights and adjustable VAc contents compared with the normal solvent 1,4-dioxane, due to the hydrogen-bonding interaction. The thermal properties of the obtained copolymers with different compositions were characterized.


 Please wait while we load your content...
Please wait while we load your content...