Synthesis of microporous organic polymers via radical polymerization of fumaronitrile with divinylbenzene†
Abstract
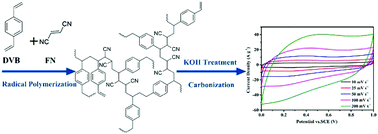
To circumvent the intractable disadvantages of a hyper-cross-linked strategy based on the Friedel–Crafts reaction, a new type of microporous organic polymer (MOP) has been successfully prepared using fumaronitrile and divinylbenzene via alternating radical polymerization. The obtained MOPs exhibit a maximum surface area of 805 m2 g−1 and excellent thermochemical stability. By pyrolyzing the copolymer precursor, a rich nitrogen-doped porous carbon material can be produced, which possesses a specific surface area of 1450 m2 g−1 with a CO2 uptake of 30 wt% at 273 K. The porous carbon also shows a high specific capacitance of 330 F g−1 at a current density of 1.0 A g−1 and a good cycling stability of 96.8% retention after 8000 cycles in a three electrode system. The unique synthesis strategy inspires researchers to seek novel building blocks for the scaled-up preparation of MOPs and the resulting porous carbon has promising application for gas adsorption and energy storage.


 Please wait while we load your content...
Please wait while we load your content...