A preorganized dual H-bond donor promotes benzoic acid active in the polymerization of δ-valerolactone†
Abstract
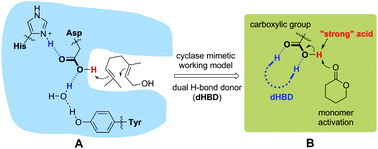
Ring-opening polymerization (ROP) of lactones catalyzed by a (super)strong Brønsted acid offers a valuable approach to important biodegradable aliphatic polyesters. However, the need for a mild acidic catalyst in ROP has been long sought after but unmet. Here, we describe a truly weak Brønsted acid, benzoic acid, in combination with a dual H-bond donor (dHBD), which catalyzes the ROP of δ-valerolactone (VL) in solution at room temperature. A unique preorganized sulfonyl guanidine type of dHBD, 3-amino-1,2,4-benzothiadiazine-1,1-dioxide (ABTD), proves optimal to work with benzoic acid as a cocatalyst, promoting benzoic acid activity in the ROP of VL. Poly-δ-valerolactones of predictable molecular weights (from 2.13 to 9.33 kg mol−1) and narrow dispersities (Đ ≤ 1.16) are synthesized. The controlled character of the ROP is verified by NMR, SEC, and MALDI-ToF MS measurements. NMR titration experiments imply that ABTD preferentially binds with benzoic acid, and the benzoic acid/ABTD pair protonates the VL monomer. Weak benzoic acid is not weak for the first time in the efficient cationic ROP of VL by the protonation mechanism.


 Please wait while we load your content...
Please wait while we load your content...