Catechol- and ketone-containing multifunctional bottlebrush polymers for oxime ligation and hydrogel formation†
Abstract
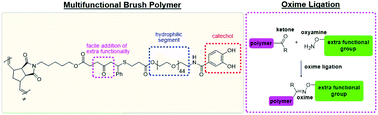
We report the synthesis of a highly-functional macromonomer, and subsequent crosslinkable poly(ethylene glycol) (PEG)-based bottlebrush polymers prepared via graft-through ring-opening metathesis polymerization (ROMP). This work makes two important additions to the repertoire of bottlebrush polymers: (1) the first example of a catechol-derived brush adhesive relying on an internal ketone for post-polymerization modification; and (2) the post-polymerization modification is utilized to greatly affect the mechanical properties of the bulk material through oxime functionalization and subsequent metal-free click chemistry beginning from the innocuous, and easily introduced, internal ketone. This form of oxime ligation was demonstrated with a series of oxyamines, with degrees of functionalization on the range of 18–100%, while introducing further functionality in the form of azide groups, which were rapidly converted to corresponding triazoles via metal-free click chemistry using the crosslinker (1R, 8S, 9S)-bicyclo[6.1.0]non-4-yn-9-ylmethyl (BCN) bisfunctionalized PEG (Mn = ∼6000 g mol−1). Under these conditions, the transformation from soluble polymer solution to soft hydrogel occurs within minutes. The polymer described herein stands testament to the ability of modularly-designed macromonomers to dictate the structure and properties of downstream materials in a predicable fashion.

 Please wait while we load your content...
Please wait while we load your content...