Well-defined triblock copolymers of polyethylene with polycaprolactone or polystyrene using a novel difunctional polyhomologation initiator†
Abstract
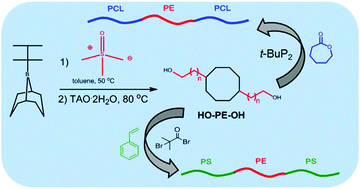
α,ω-Dihydroxy polyethylene was synthesized by polyhomologation of dimethylsulfoxonium methylide with 9-thexyl-9-BBN (9-BNN: 9-borabicyclo[3.3.1]nonane), a novel difunctional initiator produced from 9-BBN and 2,3-dimethylbut-2-ene, with two active and one blocked sites, followed by hydrolysis/oxidation. The terminal hydroxy groups were either used directly as initiators, in the presence of 1-tert-butyl-2,2,4,4,4-pentakis(dimethylamino)-2λ5,4λ5-catenadi(phosphazene) (t-BuP2), for the ring opening polymerization of ε-caprolactone to afford polycaprolactone-b-polyethylene-b-polycaprolactone (PCL-b-PE-b-PCL) or after transformation to atom transfer radical polymerization initiating sites, for the polymerization of styrene to produce polystyrene-b-polyethylene-b-polystyrene (PSt-b-PE-b-PSt) triblock copolymers. Molecular characterization by 11B, 13C and 1H NMR as well as FTIR, and high temperature GPC (HT-GPC) confirmed the well-defined nature of the synthesized new difunctional initiator and triblock copolymers. Differential scanning calorimetry was used to determine the melting points of PE and PCL.


 Please wait while we load your content...
Please wait while we load your content...