Structure/property relationships in copolymers comprising renewable isosorbide, glucarodilactone, and 2,5-bis(hydroxymethyl)furan subunits†
Abstract
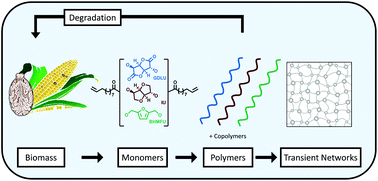
Carbohydrates and their derivatives have great potential as building blocks for the development of renewable materials that are cost and performance competitive with conventional petroleum-based materials. To this end, the rigid carbohydrate-derived diols, glucarodilactone and isosorbide were functionalized with a castor oil derivative, 10-undecenoic acid, forming α,ω-dienes suitable for acyclic diene metathesis (ADMET) polymerization. Equimolar copolymerizations of these two monomers, glucarodilactone undecenoate (GDLU) and isosorbide undecenoate (GDLU), transform brittle homopolymers into elastic materials with shape memory capabilities. To understand the source of these properties, a series of copolymers consisting of a range of GDLU and IU compositions (100 : 0, 77 : 23, 76 : 24, 52 : 48, 48 : 52, 18 : 82, 0 : 100 mol percent GDLU to IU) was synthesized. The material and chemical properties were characterized by uniaxial tensile testing, small-amplitude oscillatory shear rheology, X-ray scattering and hydrolytic degradation testing. Small compositional changes in this family were shown to have a drastic impact on the observed mechanical performance and degradability of these materials. Rheological measurements of GDLU-containing copolymers found evidence for the presence of transient networking within these materials. We posit that the transient network within this material is responsible for the elasticity and shape memory abilities of the GDL-containing materials. In confirmation of these properties, a novel α,ω-diene 2,5-bis(hydroxymethylfuran) undecenoate (BHMFU) was developed and served as a direct replacement for either GDLU or IU in copolymerizations. The replacement of GDLU resulted in a total loss of the elasticity and shape memory of the copolymers, supporting the role of GDLU in the mechanical performance.


 Please wait while we load your content...
Please wait while we load your content...