Facile one-pot access to π-conjugated polymers via sequential bromination/direct arylation polycondensation†
Abstract
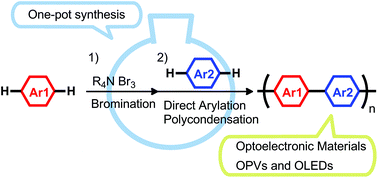
The synthesis of π-conjugated polymers starting from unfunctionalized aromatic monomers via sequential bromination/direct arylation polycondensation was investigated. The developed protocol provides a step-economical access to the polymers in a one-pot fashion, without the need for prior preparation and purification of dibrominated aromatic monomers or organometallic monomers. Benzyltrimethylammonium tribromide was effective for the bromination of 10-(2-octyldodecyl)phenothiazine and 4,4′-didodecyl-2,2′-bithiophene, and the obtained dibrominated aromatic monomers were used for the subsequent direct arylation polycondensation without isolation and purification. The direct arylation polycondensation reaction yielded the corresponding donor–acceptor-type π-conjugated polymers in moderate to good yields. The sequential protocol was also applicable to the synthesis of poly(3-hexylthiophene-2,5-diyl) from 3-hexylthiophene. The obtained polymers served as semiconducting materials in organic light-emitting diodes and organic photovoltaics.


 Please wait while we load your content...
Please wait while we load your content...