High solids content nitroxide mediated miniemulsion polymerization of n-butyl methacrylate†
Abstract
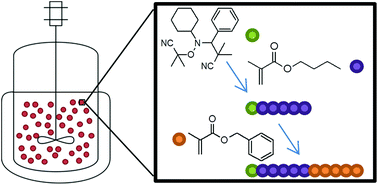
The synthesis of poly(n-butyl methacrylate) by nitroxide mediated miniemulsion polymerization is described using the alkoxyamine 3-(((2-cyanopropan-2-yl)oxy)(cyclohexyl)amino)-2,2-dimethyl-3-phenylpropanenitrile. Polymerizations are successfully conducted to high conversions with high solids content (up to 52%) and molecular weight up to 60 000 g mol−1, whilst maintaining reasonable control of the reaction. It is shown that by performing the polymerization in aqueous media, problems that arise when targeting high molecular weights in bulk/solution due to build-up of nitroxide can be overcome. The ability to chain extend the resulting polymers is demonstrated and opens the possibility for synthesis of a wide range of macromolecular structures in dispersed media.


 Please wait while we load your content...
Please wait while we load your content...