Photo-responsive thiol–ene networks for the design of switchable polymer patterns†
Abstract
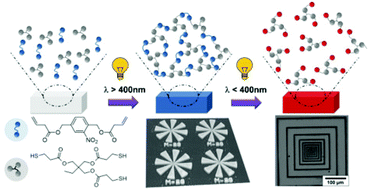
This study highlights the photo-patternable degradation of thiol–ene networks by combining the photoisomerization of o-nitrobenzyl ester (o-NBE) groups with the advantages of a photo-induced “click” reaction. Photo-responsive alkene monomers with o-NBE links are synthesized and crosslinked with selected multi-functional thiols via radical-mediated thiol–ene chemistry. By pursuing an initiation with visible light (λ > 400 nm), fast and efficient photopolymerization of the monomers is obtained under mild conditions without inducing the photocleavage of the o-NBE links. The UV induced (λ < 400 nm) photo-degradation involving a cleavage of covalent links is controlled by the functionality of the thiol crosslinker and evidenced by sol–gel analysis. The wavelength dependent changes in solubility are then applied for the design of polymer patterns which are inscribed in thin polymer films by photolithographic processes and characterized by microscopy techniques. Negative tone patterns are achieved by the radical-mediated photopolymerization of the monomers upon visible light exposure. Subsequent UV irradiation allows switching to positive tone patterns by spatially controlled photodegradation of the thiol–ene networks and surface relief patterns with a resolution of 4 μm are accomplished.

 Please wait while we load your content...
Please wait while we load your content...