Sequence regulation in the living anionic copolymerization of styrene and 1-(4-dimethylaminophenyl)-1-phenylethylene by modification with different additives†
Abstract
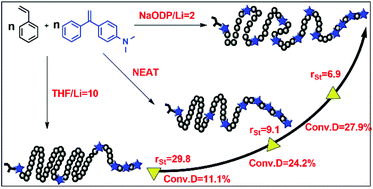
The amino-functionalized DPE derivative 1-(4-dimethylaminophenyl)-1-phenylethylene (DPE-NMe2) was used in a living anionic copolymerization with styrene (St), and the corresponding sequence structures of the copolymers were investigated using the sequence determination method. The copolymerization of St and DPE-NMe2 was neatly carried out at 24 °C, and a gradient sequence structure was obtained, with the DPE-NMe2 units incrementally distributed along the copolymer chains. For the facile regulation of the sequence distribution of the DPE-NMe2 units in the copolymer chains, the additives tetrahydrofuran (THF) or sodium 2,3-dimethylpentan-3-olate (NaODP) were introduced into the copolymerization reactions. The detailed investigations of the conversions, reactivity ratios, and sequence structures indicated that THF had a restraining effect on the incorporation of DPE-NMe2 units into the copolymer chains, and the variations in the corresponding conversions and reactivity ratios also demonstrated this tendency. In addition, a stimulatory effect was observed in the copolymerization when NaODP was used as an additive. On comparing the sequence distribution of DPE-NMe2 units in the chains among these three copolymerization reactions, it was found that NaODP promoted a higher incorporation of DPE-NMe2 into the chains than the other two conditions. This conclusion was also confirmed by the kinetic results. The values of the reactivity ratios were 9.1, 29.8 and 6.9 for the neat, THF, and NaODP conditions, respectively. For the neat, THF and NaODP conditions, the apparent reactivity ratios for styrene (KSt) were calculated to be 0.0548 min−1, 0.209 min−1 and 0.107 min−1, respectively, while the apparent reactivity ratios for DPE-NMe2 (KDPE) were calculated to be 1.82 × 10−3 min−1, 7.42 × 10−4 min−1 and 6.17 × 10−3 min−1, respectively.


 Please wait while we load your content...
Please wait while we load your content...