Synthesis of midblock-quaternized triblock copolystyrenes as highly conductive and alkaline-stable anion-exchange membranes†
Abstract
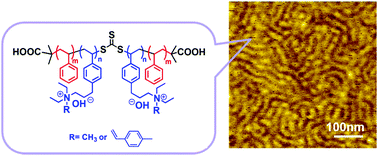
N,N-Diethyl-3-(4-vinylphenyl) propan-1-amine (DVPPA), a new monomer bearing a trialkyl amine group, provides versatile functionality to polystyrene (PS) PSm-PDVPPA2n-PSm triblock copolymers. A controlled synthetic strategy minimized chain transfer reactions while enabling the preparation of high molecular weight ABA triblock copolymers with relatively narrow (1.30–1.35) polydispersity indexes (PDIs) via reversible addition–fragmentation chain transfer (RAFT) polymerization between the DVPPA monomer and a difunctional PS macroinitiator. The presence of a tertiary amine functionality and its quaternized derivatives in the central blocks of the triblock copolymers afforded midblock-quaternized triblock co-PSs bearing pendent quaternary ammonium groups. These groups linked to the aromatic backbone with an alkyl spacer chain (3 carbon atoms) serving as steric hindrance shielding ionic centers. This polymer electrolyte with a “side-chain-type” polymer structure was designed by combining phase-separation architecture domains (confirmed by small-angle X-ray scattering (SAXS) and atomic force microscopy (AFM) analyses) and a crosslinking strategy with the aim to improve both the mechanical and alkaline stability properties. The side chain of this type of polyelectrolyte was incorporated by one quaternary ammonium group terminated with or without a crosslinkable unsaturated bond. After being simply heated at 80 °C for more than 18 h, crosslinked anion-exchange membranes (AEMs) exhibited enhanced tensile strength values (23.8–20.7 MPa) several times higher than those of conventional AEMs. An ionic conductivity of 26.1 mS cm−1 combined with low water uptake (16.5%) and swelling ratio (7.4%) values were achieved at room temperature for highly crosslinked membranes. Moreover, the membranes retained approximately up to 93.0% of their initial high hydroxide conductivity after the alkaline stability test (10 M aqueous NaOH, 80 °C, 20 d), thereby indicating excellent alkaline stability. Furthermore, in view of the good dimensional stability and satisfying mechanical properties of the resulting membranes, this approach that combines phase-separation architectures and crosslinking perfectly overcomes the obstacles blocking the development of AEMs, thereby having a great potential for practical applications.


 Please wait while we load your content...
Please wait while we load your content...