Reduction- and thermo-sensitive core-cross-linked polypeptide hybrid micelles for triggered and intracellular drug release†
Abstract
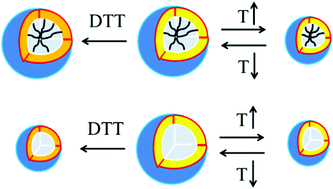
The dynamic instability, premature burst drug release, and lack of intracellular stimuli-sensitivity of current polymeric nanocarriers still hinder them from potential clinical applications. To address these challenges, a novel type of reduction- and thermo-sensitive core-cross-linked polypeptide hybrid micelle (CCM) was developed. The trimethoxysilane-terminated and disulfide-bond-centered polyglutamate with pendant diethylene glycol was synthesized from ring-opening polymerization followed by an amino-epoxy ring-opening reaction. FT-IR, 1H NMR, and thermo-gravimetric analyses verified the formation of Si–O–Si cross-linking networks in the CCMs, which have an approximately 6.6-fold lower critical aggregation concentration than the non-cross-linked counterparts. Their size, morphology and dual stimuli-sensitivity were fully characterized by DLS, TEM, and AFM. The CCMs presented a similar thermo-reversibility to non-cross-linked ones; but their size and morphology remained unchanged in 10 mM DTT at 37 °C while non-cross-linked micelles reassembled into smaller ones. The cross-linking effect in the CCMs greatly attenuated the premature burst-release behavior compared to the non-cross-linked counterparts although a reduction-triggered accelerating drug release profile was similarly observed. Furthermore, flow cytometry and CLSM confirm that the anticancer drug doxorubicin-loaded CCMs can efficiently enter the HeLa cells. Hence, this work establishes a facile platform for the fabrication of stimuli-sensitive silica-cross-linked polypeptide hybrid micelles, which hold promise to overcome the above-mentioned challenges for advanced drug delivery systems.


 Please wait while we load your content...
Please wait while we load your content...