Sunlight decatungstate photoinduced trifluoromethylations of (hetero)aromatics and electron-poor olefins†
Abstract
Tetrabutylammonium decatungstate has been exploited to photoinduce the trifluoromethylation of (hetero)aromatics and electron-poor olefins. The process made use of the cheap Langlois’ reagent (CF3SO2Na) and occurred under sunlight irradiation in an acetonitrile/water mixture. The trifluoromethylation of (hetero)aromatics required the use of potassium persulfate as an additive and the mono- or bis-trifluoromethylated adducts were obtained, depending on the actual reaction conditions and the chosen substrate.
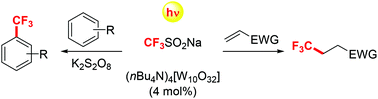


 Please wait while we load your content...
Please wait while we load your content...