Photoreactions of 2-(furan-2-yl)-3-hydroxy-4H-chromen-4-one and 3-hydroxy-2-(thiophene-2-yl)-4H-chromen-4-one using cyclohexane and acetonitrile as solvents†
Abstract
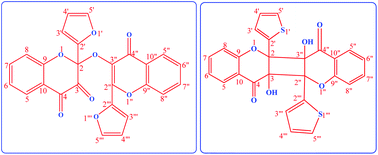
Photolysis of the titled chromenones was carried out at their longest absorption band (∼360 nm) using cyclohexane (CH) and acetonitrile (ACN) as solvents, in both aerated and de-aerated solutions. Different dimeric photoproducts were formed with both chromenones in aerated solutions. On photolysing 2-(furan-2-yl)-3-hydroxy-4H-chromen-4-one (FHC) in aerated cyclohexane, 2-(furan-2-yl)-2-{[2-(furan-2yl)-4-oxo-4H-chromen-3-yl]oxy}-2H-chromene-3,4-dione (a dehydrodimer) was formed, and on photolysing 3-hydroxy-2-(thiophene-2-yl)-4H-chromen-4-one (THC) in aerated ACN, a different dimeric product was isolated and identified. The corresponding 3-aryl-3-hydroxy-1,2-indandiones were also detected with FHC in ACN and with THC in CH, in addition to the dimeric products in both cases. On the other hand, in the de-aerated solutions, only the corresponding 1,2-indandiones were detected. 3-(Furan-2-yl)isobenzofuran-1(3H)-one as a secondary product was also detected with FHC in both solvents. An attempt was made to isolate the spectra of the photoproducts in situ. Excited State Intramolecular Proton Transfer (ESIPT) and Excited State Intramolecular Charge Transfer (ESICT) processes complicate the photodynamics of the reaction, making it difficult to predict the mechanisms of the photoreactions. However, tentative mechanisms have been proposed for the formation of the photoproducts.

 Please wait while we load your content...
Please wait while we load your content...