A benzimidazole-based chemodosimeter for the fluorometric detection of Zn and Cu via 1,5 proton shifts and C–N bond cleavage†
Abstract
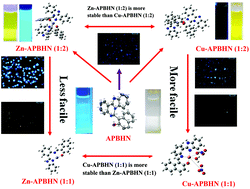
Here, we report the design and synthesis of the fluorescent probe APBHN, which was derived from 2-(1H-benzo[d]imidazol-2-yl)benzenamine and is capable of detecting intracellular Zn and Cu ions in the micromolar range. Single-crystal X-ray analysis revealed that the structure of the ligand comprises a fused cyclic system with a pendent naphthol moiety. With the addition of Zn and Cu ions the inherent fluorescence behaviour of the ligand APBHN is perturbed via a chemodosimetric change that involves a 1,5 proton shift followed by C–N bond cleavage. Upon detailed analysis, it was found that the ligand forms 1 : 1 and 1 : 2 (metal to ligand) complexes with the corresponding metal ions. The detection limits of Zn2+ and Cu2+ were 5.59 μM and 0.148 μM, respectively, with APBHN, which are lower than the WHO guidelines (76 μM for Zn2+ and 31.5 μM for Cu2+) for drinking water. Moreover, APBHN could be used as a practical, visible colorimetric test kit for both Zn2+ and Cu2+. APBHN can efficiently detect Zn2+ and Cu2+ in liver carcinoma cells with insignificant cytotoxicity.


 Please wait while we load your content...
Please wait while we load your content...