Synthesis of 2,2-bis(pyridin-2-yl amino)cyclobutanols and their conversion into 5-(pyridin-2-ylamino)dihydrofuran-2(3H)-ones†
Abstract
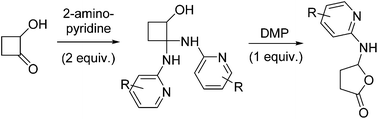
A two-step protocol is presented for the preparation of 5-(pyridin-2-ylamino)dihydrofuran-2(3H)-ones from 2-hydroxycyclobutanone and some 2-aminopyridines via a catalyst-free synthesis of 2,2-bis(pyridin-2-ylamino)cyclobutanols followed by Dess–Martin periodinane mediated ring expansion.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...