Elemental sulfur mediated 2-substituted benzothiazole formation from 2-aminobenzenethiols and arylacetylenes or styrenes under metal-free conditions†
Abstract
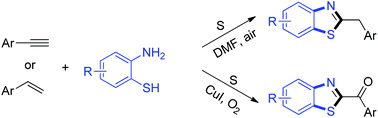
An oxidative cyclization of 2-aminothiophenols and arylacetylenes or styrenes for the synthesis of 2-alkylbenzothiazoles and 2-acylbenzothiazoles has been developed. Elemental sulfur was used as the effective oxidant to give the corresponding product in good yield under metal-free conditions.


 Please wait while we load your content...
Please wait while we load your content...