Synthesis, computational, and spectroscopic analysis of tunable highly fluorescent BN-1,2-azaborine derivatives containing the N-BOH moiety†
Abstract
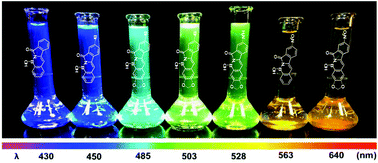
Nine new polycyclic aromatic BN-1,2-azaborine analogues containing the N-BOH moiety were synthesized using a convenient two-step, one-pot procedure. Characterization of the prepared compounds show the luminescence wavelength and the quantum yields of the azaborines were tunable by controlling the power and location of the donor and acceptor substituents on the chromophore. UV-visible spectroscopy and density functional theory (DFT) computations revealed that the addition of electron-donating moieties to the isoindolinone hemisphere raised the energy of the HOMO, resulting in the reduction of the HOMO–LUMO gap. The addition of an electron-accepting moiety to the isoindolinone hemisphere and an electron-donating group to the boronic acid hemisphere decreased the HOMO–LUMO gap considerably, leading to emission properties from partial intramolecular charge transfer (ICT) states. The combined effect of an acceptor on the isoindolinone side and a donor on the boronic acid side (strong acceptor–π-donor) gave the most red-shifted absorption. The polycyclic aromatic BN-1,2-azaborines emitted strong fluorescence in solution and in the solid-state with the largest red-shifted emission at 640 nm and a Stokes shift of Δλ = 218 nm, or Δν = 8070 cm−1.


 Please wait while we load your content...
Please wait while we load your content...