A low-temperature, photoinduced thiol–ene click reaction: a mild and efficient method for the synthesis of sugar-modified nucleosides†
Abstract
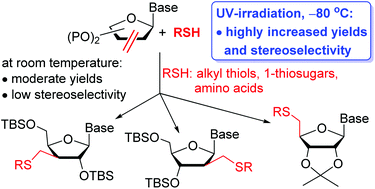
Sugar-modified nucleosides are prime synthetic targets in anticancer and antiviral drug development. Radical mediated thiol–ene coupling was applied for the first time on nucleoside enofuranoside derivatives to produce a broad range of thio-substituted D-ribo, -arabino, -xylo and L-lyxo configured pyrimidine nucleosides. In contrast to the analogous reactions of simple sugar exomethylenes, surprisingly, hydrothiolation of nucleoside alkenes under the standard conditions of various initiation methods showed low to moderate yields and very low stereoselectivity. Optimizing the reaction conditions, we have found that cooling the reaction mixture has a significant beneficial effect on both the conversion and the stereoselectivity, and UV-light initiated hydrothiolation of C2′-, C3′- and C4′-exomethylene derivatives of nucleosides at −80 °C proceeded in good to high yields, and, in most cases, in excellent diastereoselectivity. Beyond the temperature, the solvent, the protecting groups on nucleosides and, in some cases, the configuration of the thiols also affected the stereochemical outcome of the additions. The anomalous L-lyxo diastereoselectivity observed upon the addition of 1-thio-β-D-gluco- and galactopyranose derivatives onto C4′,5′-unsaturated uridines is attributed to steric mismatch between the D-ribo C4′-radical intermediates and the β-configured 1-thiosugars.


 Please wait while we load your content...
Please wait while we load your content...