AgOTf-catalyzed sequential synthesis of 4-isoquinolones via oxidative ring opening of aziridines and aza-Michael addition†
Abstract
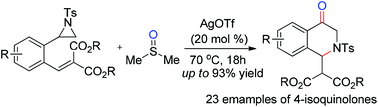
An efficient AgOTf-catalyzed sequential reaction involving the oxidative ring-opening of aziridines by DMSO and aza-Michael addition has been developed. A series of 2,3-dihydro-4(1H)-isoquinolones were afforded in moderate to good yields by the formation of one new C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond and one new C–N bond. The features of this sequential reaction include high bonding efficiency, use of a catalytic amount of catalysts, a broad substrate scope and mild conditions. This methodology provides a good choice for constructing the libraries of 2,3-dihydro-4(1H)-isoquinolones.
O bond and one new C–N bond. The features of this sequential reaction include high bonding efficiency, use of a catalytic amount of catalysts, a broad substrate scope and mild conditions. This methodology provides a good choice for constructing the libraries of 2,3-dihydro-4(1H)-isoquinolones.


 Please wait while we load your content...
Please wait while we load your content...