8-Oxo-7,8-dihydro-2′-deoxyguanosine and abasic site tandem lesions are oxidation prone yielding hydantoin products that strongly destabilize duplex DNA†
Abstract
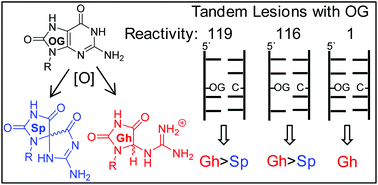
In DNA, 2′-deoxyguanosine (dG) is susceptible to oxidative modification by reactive oxygen species (ROS) yielding many products, one of which is 8-oxo-7,8-dihydro-2′-deoxyguanosine (dOG). Interestingly, dOG is stable but much more labile toward oxidation than dG, furnishing 5-guanidinohydantoin-2′-deoxyribose (dGh) that is favored in the duplex context or spiroiminodihydantoin-2′-deoxyribose (dSp) that is favored in the oxidation of single-stranded contexts. Previously, exposure of DNA to ionizing radiation found ∼50% of the dOG exists as a tandem lesion with an adjacent formamide site. The present work explored oxidation of dOG in a tandem lesion with a THF abasic site analog (F) that models the formamide on either the 5′ or 3′ side. When dOG was in a tandem lesion, both dGh and dSp were observed as oxidation products. The 5′ versus 3′ side in which F resided influenced the stereochemistry of the dSp formed. Further, tandem lesions with dOG were found to be up to two orders of magnitude more reactive to oxidation than dOG in an intact duplex. When dOG is in a tandem lesion it is up to fivefold more prone to formation of spermine cross-links during oxidation compared to dOG in an intact duplex. Lastly, dOG, dGh, and each dSp diastereomer were synthesized as part of a tandem lesion in a duplex DNA to establish that dOG tandem lesions decrease the thermal stability by 12–13 °C, while dGh or either dSp diastereomer in a tandem lesion decrease the stability by >20 °C. The biological consequences of these results are discussed.
- This article is part of the themed collection: Nucleic Acid Modifications


 Please wait while we load your content...
Please wait while we load your content...