Laser flash photolysis of nanocrystalline α-azido-p-methoxy-acetophenone†
Abstract
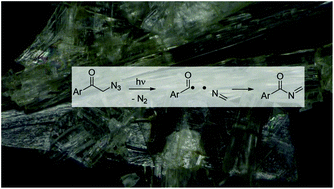
Irradiation of nanocrystals of azide 1 results in a solid-to-solid reaction that forms imine 2 in high chemical yield. In contrast, solution photolysis of azide 1 yields a mixture of products, with 7 as the major one. Laser flash photolysis (LFP) of a nanocrystalline suspension of azide 1 in water shows selective formation of benzoyl radical 4 (λmax ∼ 400 nm), which is short-lived (τ = 833 ns) as it intersystem crosses to form imine 2. In comparison, LFP of azide 1 in methanol reveals the formation of triplet alkylnitrene 10 (λmax ∼ 340 nm). The selectivity observed in the solid-state is related to stabilization of the triplet ketone with (n,π*) configuration by the crystal lattice, which results in α-cleavage being favored over triplet energy transfer to the azido chromophore. Both the solid-state and solution reaction mechanisms are further supported by density functional theory calculations. Thus, laser flash photolysis has been used to effectively elucidate the medium dependent reaction mechanisms of azide 1.
- This article is part of the themed collections: Celebrating excellence in research: women of organic chemistry and Mechanistic Aspects of Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...