An aryne-based three-component access to α-aroylamino amides†
Abstract
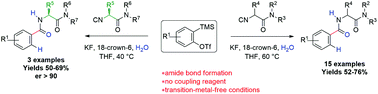
Aryne chemistry has recently received widespread attention and isocyanides have been reported as efficient nucleophilic partners in a set of multicomponent transformations. In this study, we demonstrate that tertiary α-monosubstituted α-isocyanoacetamides are efficaciously coupled with water and benzyne to offer a direct and metal-free access to densely functionalized α-benzoylamino amides, without competing with the intramolecular cyclization to 5-aminooxazoles. Despite the formation of the aryl anion as a key intermediate, the reaction displays a stereoconservative course, allowing for the preparation of enantiomerically pure α-benzoylamino amides. Finally, the synthetic utility of the reported MCR was exemplified by the preparation of proglumide, a cholecystokinin antagonist.


 Please wait while we load your content...
Please wait while we load your content...