Insight into the deprotonation at the half-equivalence point of (thio)amido-benzimidazoles in the presence of anions†
Abstract
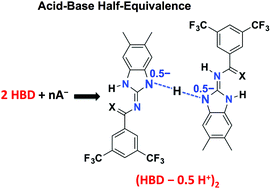
Three crystallographic structures highlight the acid–base half-equivalence point of hydrogen-bond donor (thio)amido-benzimidazoles induced by fluoride or benzoate salts with concomitant hydrogen-bonding and deprotonation as a merged synergic process.


 Please wait while we load your content...
Please wait while we load your content...