Aerobic thiyl radical addition/cyclization of N-methacryloyl benzamides for the synthesis of isoquinoline-1,3(2H,4H)-dione derivatives†
Abstract
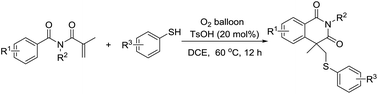
A highly effective oxidative thiyl radical addition/cyclization of N-methacryloylbenzamides was explored using dioxygen as the sole terminal oxidant without the use of precious and/or toxic transition-metal catalysts. This method provides convenient access to a variety of useful sulfide-containing 4,4-disubstituted isoquinoline-1,3-diones by constructing C–S and C–C bonds in one step.


 Please wait while we load your content...
Please wait while we load your content...