Synthesis of phostones via DABCO-catalyzed bromocyclization of alkenylphosphonic acid monoesters†
Abstract
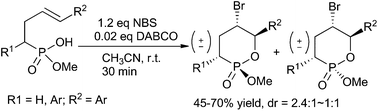
The bromocyclization of 4-aryl-3-butenylphosphonic acid monoesters could proceed smoothly and rapidly in CH3CN with 1.2 equiv. of NBS in the presence of 0.02 equiv. of DABCO at room temperature, giving exclusively the six-membered ring bromophostones with high endo regioselectivity but poor diastereoselectivity. The diastereomers were separated and their relative configurations were determined based on their NMR analysis and X-ray crystallography. Furthermore, we preliminarily demonstrated that the asymmetric bromocyclization of these kinds of substrates was possible.


 Please wait while we load your content...
Please wait while we load your content...