Carboxylate isosteres for caspase inhibitors: the acylsulfonamide case revisited†
Abstract
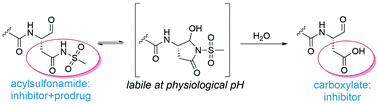
As part of an ongoing effort to discover inhibitors of caspase-1 with an optimized selectivity and biopharmaceutical profile, acylsulfonamides were explored as carboxylate isosteres for caspase inhibitors. Acylsulfonamide analogues of the clinically investigated caspase-1 inhibitor VRT-043198 and of the pan-caspase inhibitor Z-VAD-CHO were synthesized. The isostere-containing analogues with an aldehyde warhead had inhibitory potencies comparable to the carboxylate references. In addition, the conformational and tautomeric characteristics of these molecules were determined using 1H- and 13C-based NMR. The propensity of acylsulfonamides with an aldehyde warhead to occur in a ring-closed conformation at physiological pH significantly increases the sensitivity to hydrolysis of the acylsulfonamide moiety, yielding the parent carboxylate containing inhibitors. These results indicate that the acylsulfonamide analogues of the aldehyde-based inhibitor VRT-043198 might have potential as a novel type of prodrug for the latter. Finally, inhibition of caspase 1 and 11 mediated inflammation in mouse macrophages was found to correlate with the potencies of the compounds in enzymatic assays.


 Please wait while we load your content...
Please wait while we load your content...