Discovery of 2-(pyridin-2-yl)aniline as a directing group for the sp2 C–H bond amination mediated by cupric acetate†
Abstract
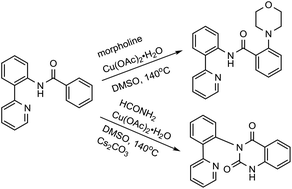
2-(Pyridin-2-yl) aniline was designed as a new, removable directing group in promoting C–H amination mediated by cupric acetate. Employing this auxiliary, the β-C(sp2)–H bonds of benzamide derivatives can be effectively aminated with a variety of amines in moderate to good yields with good functional group tolerance in air. In addition, the quinazolinone derivatives were isolated from the reaction mixture of N-(2-(pyridin-2-yl)phenyl)benzamide with formamide or 5-nitroindole. The corresponding mechanism is discussed. These results indicate that 2-(pyridine-2-yl)aniline can serve as a directing group.


 Please wait while we load your content...
Please wait while we load your content...