Diphenylacrylonitrile-connected BODIPY dyes: fluorescence enhancement based on dark and AIE resonance energy transfer†
Abstract
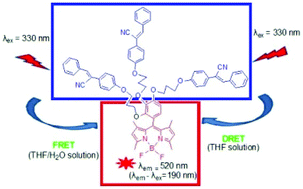
This study focuses on the construction of novel diphenylacrylonitrile-connected BODIPY dyes with high fluorescence in both solution and an aggregated state by combining DRET and FRET processes in a single donor–acceptor system. The first BODIPY derivatives with one, two, or three AIE-active diphenylacrylonitrile groups were designed and synthesized in moderate yields. Strong fluorescence emissions were observed in the THF solution under excitation at the absorption wavelength of non-emissive diphenylacrylonitrile chromophores, implying the existence of the DRET process between the dark diphenylacrylonitrile donor and the emissive BODIPY acceptor. In the THF/H2O solution, the fluorescence intensity of the novel BODIPY derivatives gradually increased under excitation at the absorption wavelength of diphenylacrylonitrile chromophores, suggesting a FRET process between diphenylacrylonitrile and BODIPY moieties. A greater number of diphenylacrylonitrile units led to higher energy-transfer efficiencies. The pseudo-Stokes shift for both DRET and FRET processes was as large as 190 nm.


 Please wait while we load your content...
Please wait while we load your content...