Supramolecular control of heme binding and electronic states in multi-heme peptide assemblies†
Abstract
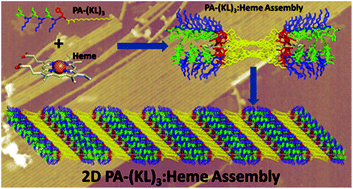
Nature guides the flow of electrons in biological systems with the assistance of multi-heme proteins called cytochromes. In an effort to understand natures approach to developing electronic systems, three peptides that are compositionally identical but sequentially distinct have been designed to study the impact of morphology and hydrophobicity on heme coordination and function.
- This article is part of the themed collection: Peptide Materials


 Please wait while we load your content...
Please wait while we load your content...