Stabilizing bubble and droplet interfaces using dipeptide hydrogels
Abstract
Hydrophobic dipeptide molecules can be used to create interfacial films covering bubbles and droplets made from a range of oils. At high pH, the dipeptide molecules form micelles which transform into a hydrogel of fibres in response to the addition of salt. We characterize the properties of the hydrogel for two different salt (MgSO4) concentrations and then we use these gels to stabilize interfaces. Under high shear, the hydrogel is disrupted and will reform around bubbles or droplets. Here, we reveal that at low dipeptide concentration, the gel is too weak to prevent ripening of the bubbles; this then reduces the long-term stability of the foam. Under the same conditions, emulsions prepared from some oils are highly stable. We examine the wetting properties of the oil droplets at a hydrogel surface as a guide to the resulting emulsions.
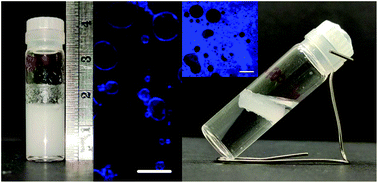
- This article is part of the themed collection: Peptide Materials


 Please wait while we load your content...
Please wait while we load your content...