Palladium-catalyzed geometrically selective hydrogenation of (Z)-trifluoromethyl alkenyl triflate: an efficient approach to (Z) or (E)-3,3,3-trifluoropropenyl derivatives†
Abstract
A Pd-catalyzed hydrogenation of (Z)-trifluoromethyl alkenyl triflate providing either (Z)- or (E)-3,3,3-trifluoropropenyl derivatives with excellent divergent geometric control in good yield is described. Catalyzed by Pd(OAc)2/PPh3, the reduction of (Z)-trifluoromethyl alkenyl triflates with HSiEt3 gave (E)-3,3,3-trifluoropropenyl derivatives, and while using HCOOH/Et3N as the reducing agent, the (Z)-isomers were obtained through an elimination/hydrogenation tandem pathway. Both transformations showed excellent geometrical selectivity.
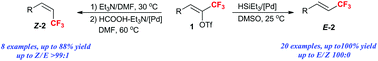


 Please wait while we load your content...
Please wait while we load your content...