Evaluation of ligand-based NMR screening methods to characterize small molecule binding to HIV-1 glycoprotein-41†
Abstract
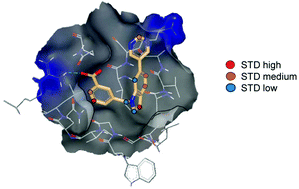
Small molecule inhibitors of glycoprotein-41 (gp41) are able to prevent HIV infection by binding to a hydrophobic pocket (HP) contained within the gp41 ectodomain, and preventing progression of fusion. There is little structural information on gp41–ligand complexes, owing to hydrophobicity of the ligands, occlusion of the HP in folded gp41 ectodomain, and failure to form crystals of complexes. Here we used an engineered gp41 ectodomain protein containing an exposed HP and a small molecule designed to bind with weak affinity to the HP. We evaluated NMR methods, including WaterLOGSY, Saturation Transfer Difference spectroscopy (STD-NMR) and 1H relaxation rate difference spectroscopy with and without target irradiation (DIRECTION) for their ability to probe complex formation and structure. WaterLOGSY was the most sensitive technique for monitoring formation of the complex. STD-NMR and DIRECTION experiments gave similar pharmacophore mapping profiles, although the low dynamic range of the DIRECTION experiment limited its discrimination and sensitivity. A unique binding pose was identified from the STD data and provided clues for future optimization. Advantages and disadvantages of the techniques are discussed. This is the first example of the use of STD for structural analysis of a gp41-small molecule complex.


 Please wait while we load your content...
Please wait while we load your content...