Natural product driven diversity via skeletal remodeling of caryophyllene β-lactam†
Abstract
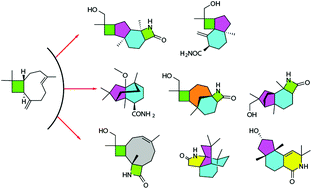
(−)-β-Caryophyllene was decorated with a privileged β-lactam motif and subsequently converted into highly diverse scaffolds via remodeling of the ring system. The structures were defined by spectroscopic data, X-ray diffraction analysis, and experimental and calculated ECD data. Compound 19 displayed the most potent activity against the rice blast fungus, while 6 had a more potent α-glucosidase inhibition than the drug acarbose. These findings demonstrate a concise protocol to exploit natural product-driven diversity.


 Please wait while we load your content...
Please wait while we load your content...