Cleavage of 1,3-dicarbonyls through oxidative amidation†
Abstract
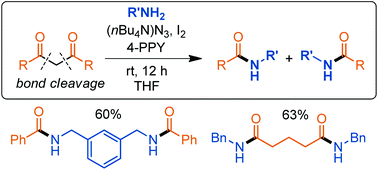
A mild and convenient protocol for the oxidative cleavage of 1,3-diketone compounds is described. Under metal-free conditions, the method converts the 1,3-dicarbonyls into amides when treated with (nBu4N)N3 and iodine in the presence of an amine at room temperature. Using this method, a range of 1,3-dicarbonyls with various structural motifs including sterically demanding substituents and ordinary functional groups were easily fragmented, and it is demonstrated that cyclic 1,3-dicarbonyls can be directly transformed into acyclic diamides through ring-opening. Initial mechanistic studies show that diazidation of the enol form is followed by nucleophilic substitution with the amine.


 Please wait while we load your content...
Please wait while we load your content...