Two-step conversion of carboxylic esters into distally fluorinated ketones via ring cleavage of cyclopropanol intermediates: application of sulfinate salts as fluoroalkylating reagents†
Abstract
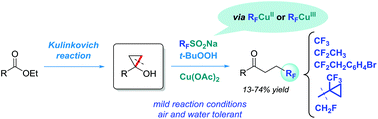
Tertiary cyclopropanols easily available from carboxylic esters have been used in the synthesis of distally fluorinated ketones. Cyclopropane ring cleavage reactions in methanol with aqueous tert-butyl hydroperoxide in the presence of a copper(II) acetate catalyst and sodium triflinate (Langlois reagent) afford β-trifluoromethyl ketones in 16–74% isolated yields. Sodium triflinate serves as a precursor of reactive trifluoromethyl copper species, enabling ring-opening trifluoromethylation, as evidenced by mechanistic studies. We also demonstrate here that other sulfinate salts, such as sodium 1,1-difluoroethanesulfinate, sodium 2-(4-bromophenyl)-1,1-difluoroethanesulfinate and sodium 1-(trifluoromethyl)cyclopropanesulfinate, can be used as fluoroalkylation reagents, resulting in the corresponding fluorinated ketones.


 Please wait while we load your content...
Please wait while we load your content...