Palladium-catalyzed C–H olefination of uracils and caffeines using molecular oxygen as the sole oxidant†
Abstract
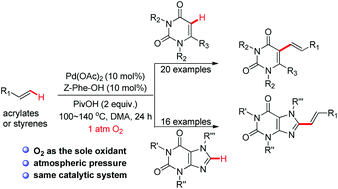
The palladium-catalyzed oxidative C–H olefination of uracils or caffeines with alkenes using an atmospheric pressure of molecular oxygen as the sole oxidant has been disclosed. This novel strategy offers an efficient and environmentally friendly method to biologically important C5-alkene uracil derivatives or C8-alkene caffeine derivatives.


 Please wait while we load your content...
Please wait while we load your content...