Pd/Cu-Catalyzed tandem head-to-tail dimerization/cycloisomerization of terminal ynamides for the synthesis of 5-vinyloxazolones†
Abstract
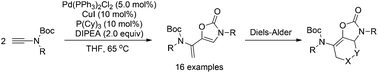
An attractive and novel methodology involving Pd/Cu-catalyzed tandem head-to-tail dimerization/cycloisomerization of terminal ynamides for the synthesis of 3,5-disubstituted oxazolones was developed. Under Pd(PPh3)2Cl2/CuI cooperative catalyzed reaction conditions, it provided efficient access to 5-vinyloxazolones with exceptional functional group tolerance and good chemoselectivity. The control experiments demonstrated that Pd(PPh3)2Cl2 serves a key role in the dimerization of terminal ynamides and shows low catalytic activity in the intramolecular cyclization. Moreover, the hetero-Diels–Alder reaction of product 5-vinyloxazolones was also described, which provided polycyclic oxazolones in good yield.


 Please wait while we load your content...
Please wait while we load your content...