Novel chemoenzymatic oxidation of amines into oximes based on hydrolase-catalysed peracid formation†
Abstract
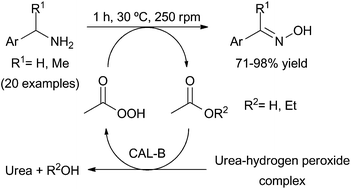
The efficient transformation of benzylamines into the corresponding oximes has been described by means of a chemoenzymatic process. This strategy is based on a two-step sequence developed in one-pot at 30 °C and atmospheric pressure. First, the formation of a reactive peracid intermediate occurs by means of a lipase-catalysed perhydrolysis reaction, and then this peracid acts as a chemical oxidising agent of the amines. A total of nine ketoximes were isolated in high purity after a simple extraction protocol (90–98% isolated yield), while for the eleven synthesised aldoximes a further column chromatography purification was required (71–82% isolated yield). In all cases excellent selectivities were attained, offering a practical method for amine oxidation in short reaction times (1 hour). The environmental impact of the process was analysed and compared with a recently published alternative chemical synthesis, finding for this metric a good E-factor value.
- This article is part of the themed collection: Biocatalysis: Natural and biologically inspired synthetic enzymes


 Please wait while we load your content...
Please wait while we load your content...