Efficient synthesis of aliphatic sulfones by Mg mediated coupling reactions of sulfonyl chlorides and aliphatic halides†
Abstract
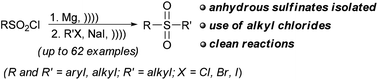
Sulfonyl chlorides were reduced to anhydrous sulfinate salts with magnesium under sonication. These sulfinates were alkylated to sulfones with alkyl chlorides in the presence of catalytic sodium iodide under sonication. A variety of aliphatic sulfones was efficiently prepared by this one-pot two-step procedure.


 Please wait while we load your content...
Please wait while we load your content...