Synthesis of alkynyl furoxans. Rare carbon–carbon bond-forming reaction on a furoxan ring†
Abstract
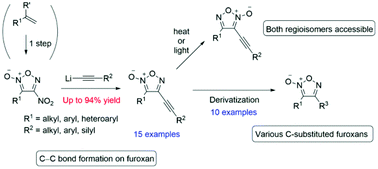
A rare carbon–carbon-bond forming reaction on a furoxan ring has been developed. The nucleophilic aromatic substitution (SNAr) reaction of 4-nitrofuroxans with alkynyl lithium proceeded with high yields, which enabled the first practical synthesis of both alkynyl furoxan regioisomers. Due to the versatility of the alkyne functional group, various derivatizations of the carbon–carbon triple bond in the afforded products were possible. Thus, this developed method is a convergent approach to a wide spectrum of carbon-substituted furoxans.


 Please wait while we load your content...
Please wait while we load your content...