One-pot construction of sterically challenging and diverse polyarylphenols via transition-metal-free benzannulation and their potent in vitro antioxidant activity†
Abstract
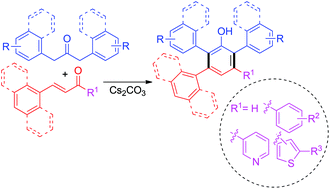
This paper reports the novel and efficient one-pot synthesis of highly functionalized polyarylphenols from readily available 1,3-diarylpropan-2-ones and chalcones or cinnamaldehyde derivatives via benzannulation under transition-metal-free and aerobic conditions. This benzannulation proceeds through cascade Michael addition/intramolecular aldol/tautomerization/oxidation. This protocol produces various tetra- and tri-aryl substituted phenols in moderate to good yield. The synthesized compounds exhibited potent antioxidant activities compared to the standard BHT.


 Please wait while we load your content...
Please wait while we load your content...