Transfer of axial chirality through the nickel-catalysed hydrocyanation of chiral allenes†
Abstract
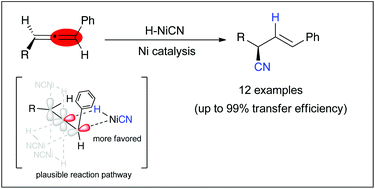
The transfer of axial chirality of allenes is beginning to be exploited as a powerful method for creating central chirality, particularly through the use of transition metal catalysis. In this communication, the transfer of axial chirality of chiral allenes via nickel-catalysed hydrocyanation is achieved through both regio- and face-selective hydronickelation as well as regioselective reductive elimination. This protocol was applied to 12 substrates and gave chiral carbonitriles with up to 97% ee. Further application to hydrocyanative cyclization using a chiral allene–yne is also presented, along with a discussion of the corresponding mechanism of racemization.


 Please wait while we load your content...
Please wait while we load your content...