Nickel-catalyzed product-controllable amidation and imidation of sp3 C–H bonds in substituted toluenes with sulfonamides†
Abstract
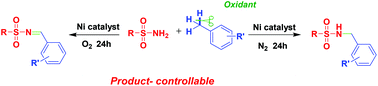
A nickel-catalyzed product-controllable imidation and amidation of sp3 C–H bonds in substituted toluenes with sulfonamides were developed. Based on the change of the reaction time and atmosphere from N2 to O2, this reaction proceeded in high yields and excellent selectivity under different conditions. Mechanistic details were also described.

 Please wait while we load your content...
Please wait while we load your content...